

News


 |
30 new projects and an increase by 70% in the incomes in the field of clinical trials. |
 |
Continuation of the cooperation with pharmaceutical market leaders (Adamed, AstraZeneca, Chiesi, Pfizer, Polpharma, Servier, Takeda) and participation in the international trials. |
 |
New contracts for non-commercial clinical trials (Medical University of Warsaw, Medical University of Wrocław, National Research Institute of Oncology). |
 |
Dozens of market and opinion research for business (Bank Gospodarstwa Krajowego, TEB Akademia, Sedlak & Sedlak, Grant Thornton, PGNiG) and over 100 completed scientific studies. |
 |
Development of the SURVGO research tool and Badanie-Opinii.pl research panel. |
 |
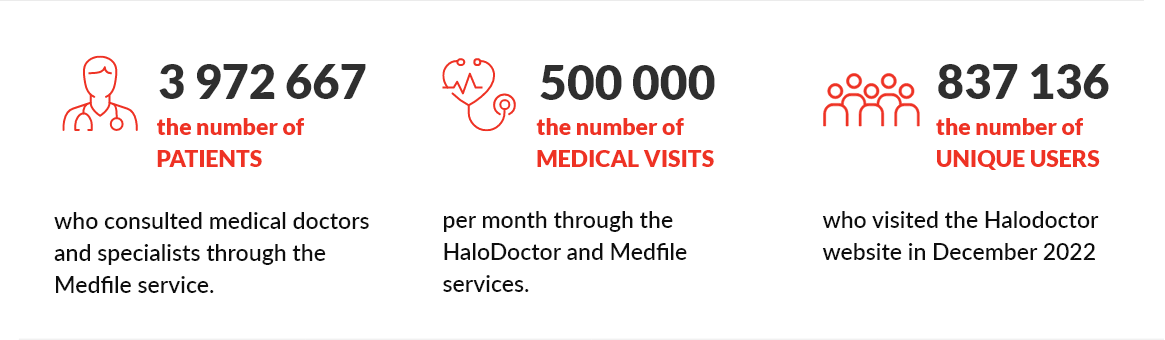
Over 10,000 medical doctors and 15 million medical doctor visits handled by the Medfile program and the HaloDoctor service. |
 |
Expansion into the Norwegian market. |
 |
Increase in employment by 25% with IT, clinical research (CRO) and marketing specialists. |
It was a successful year 2022 at Biostat!
In the field of broadly understood clinical trials, over 30 new projects appeared in Biostat's portfolio in 2022. The largest number of new projects are in specializations such as: cardiology, oncology, allergology/pulmonology, paediatrics, and gastroenterology.
Importantly, we have begun participating in important cardiological studies conducted for large pharmaceutical companies: Adamed and AstraZeneca. One of these projects has a global reach and is conducted in 9 European countries.
We have also tightened the cooperation with another leader on the pharmaceutical market - Chiesi, with which we are initiating new research projects in the field of neonatology and allergology. One of these projects is also carried out in European countries and will last until mid-2023.
Without a doubt, in 2022 we strengthened Biostat's position among CRO companies on the Polish market. We have won several tenders for the service of non-commercial clinical trials, as a result of which the recognition of Biostat's quality has significantly increased. Due to won tenders and signed contracts, 2023 promises to be very intense for us - emphasizes Edyta Klemba - Pharma Division Director at Biostat®.
Let us add that the public institutions and universities cooperating with Biostat include: Medical University of Warsaw, Medical University of Wrocław, Medical University of Gdańsk, National Research Institute of Oncology, Jagiellonian University, University Teaching Hospital in Opole, Nicolaus Copernicus University in Toruń, Śląskie Centrum Chorób Serca and Instytut Hematologii i Transfuzjologii.

> Learn more about Biostat's clinical research services
Market and opinion research, widely understood marketing research and research in the field of human resources management, including employee satisfaction surveys, are increasing in recognition and interest from customers.
In our research, we combine various research techniques - CAWI, CATI, CAPI and IDI.
In the last 12 months, we have had the pleasure of conducting research for such companies and institutions as: Bank Gospodarstwa Krajowego, National Institute of Public Health, Cracow University of Economics, TEB Academia, Sedlak & Sedlak, Grant Thornton, PGNiG, Scanmed and Lublin University of Technology.
A report on the pharmaceutical and cosmetics industry in Warsaw, commissioned by the City of Warsaw, was also an interesting and complex project implemented in 2022.
Let us emphasize that scientific research and clients in the form of universities are still a strong position in our portfolio. Last year, we carried out over 100 such studies. We have included, among others two framework agreements for the implementation of scientific research of university employees - at Lublin University of Technology and Wrocław University of Economics.
The year 2022 was also a time of development for the SurvGo website. It is a tool enabling business clients, scientists and individuals to design and carry out their own survey research, based on our knowledge and a database of over 150,000 survey respondents in the Badanie-Opinii.pl research panel.

On November 15th, 2022, the Minister of Development and Technology awarded Biostat the status of Research and Development Centre for the fourth year in a row. Thus, we found ourselves in the elite group of 63 commercial research units of this type.
We are glad that the quality and innovation of our services has been appreciated once again. It is the result of the work of our team and several hundred innovative projects implemented in the field of clinical research (CRO), market research and IT - evaluates Rafał Piszczek - Chairman of the Board at Biostat.
The past year was a time of dynamic development of our proprietary Medfile software. It is a comprehensive software for medical doctors’ offices and clinics. Medfile provides specialized e-Health services via API for entities and companies with their own office software with very little effort and time. Currently, more than 10,000 medical doctors are using it in Poland.
In 2022, we developed our software, e.g. VOIP technology and a range of services to facilitate accounting and reporting. Large teams of medical clinics have joined the group of our clients, such as: Psychomedic, Mental Path, Lower Silesian Psychotherapy Centre, Psychoklinika, Severux and Medical Magnus.
Our Halodoctor service has an increasingly strong position on the telemedicine market in Poland. In December 2022, the website was visited by 837,136 unique users. Services most often selected by patients on the HaloDoctor website are: e-doctor visit, e-prescription, e-medical leave, psychological assistance and arranging stationary visits.
Using the Medfile software and the haloDoctor service, half a million medical doctor visits are handled monthly!
W 2022 roku zawarliśmy porozumienie z norweskim partnerem strategicznym ViciData. Na mocy porozumienia rozpoczęliśmy prace nad adaptacją i wdrożeniem na rynek norweski oprogramowania dla placówek medycznych, bazującego na naszym programie MedFile.
In recent months, we have expanded our range of courses in statistics in clinical trials. Our offer is addressed both to people working in the clinical trials industry, as well as candidates for the positions of clinical trial monitors and assistants. Training is available both in the form of online training and live webinars conducted by Biostat experts.
The patronage over the edu.biostat.com.pl website was taken by the Association for Good Clinical Trials Practice in Poland.
The clinical trials and opinion polls carried out by the Biostat Research and Development Centre were noticed in 2022 by the opinion-forming media. The results of our research were cited, among others, by Medonet, Wirtualną Polskę, Rzeczpospolitą, Gazetę Wyborczą, Polską Agencję Prasową, ABC Zdrowie, Spider’s Web, Interię, or Śląski Biznes.
Due to the growing number of projects and customer portfolio in 2022, our team has grown by 25%! Among the new specialists who now support Biostat are: programmers, statisticians, data analysts, clinical trial monitors and assistants, project managers, graphic designers, journalists and customer service employees.
We have entered the new year with a rich portfolio of new and ongoing projects.
In the clinical trials industry, a new challenge is the CTIS system, which will be launched in January and will harmonize the process of submitting, assessing and supervising clinical trials in the European Union. This is a significant change for the entire research community.
Our goal is to expand our customer portfolio with partners who intend to introduce or recertify medical devices. We will also work on increasing the recognition of the Biostat brand on the European arena - announces Rafał Piszczek – the Chairman of the Board at Biostat.
In the area of market research, we will focus on research carried out for the business and science sectors, including scientific research, brand research, customer satisfaction, employee satisfaction and campaign effectiveness research.
Everything indicates that 2023 will be a period of dynamic development of telemedicine in Poland and Europe. Hence, we see broad development prospects ahead of our Medfile and HaloDoctor projects - adds Rafał Piszczek.
Kowalczyka 17
44-206 Rybnik
Poland


