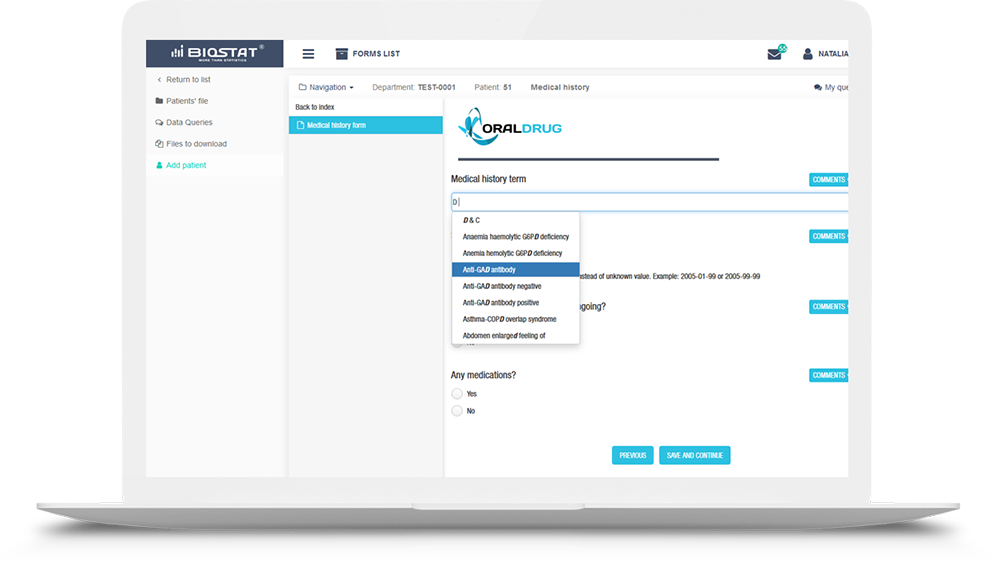
Using the license, we have equipped eCRF.bizTM system with the
MedDRA medical dictionary containing international medical terminology
used in clinical trials, clinical trial reports, data presentation and medical coding.
The use of the MedDRA dictionary in the eCRF system provides
global standardization of terminology in a regulatory-compliant
study and allows to properly classify diseases, diagnoses, symptoms, therapeutic
indications, tests, procedures, and medical history of patients.
Frequently asked questions.