Highest quality in accordance with the requirements of Philadelphia-listed journals and FDA.
We provide professional statistical analysis services for various purposes, including academic writing (PhD theses and habilitations), international publications, and business studies (such as Business Intelligence and clinical trials).
Our analyses come with a 12-month guarantee. We support you in the further stages of working on articles and in the review process.
Study Design:

Creating Statistical Analysis Plans

Sample selection and sample size calculations

Randomization

Questionnaire preparation,

eCRF development

Selection of statistical analysis methods

Selection of data collection methods

Data Collection:

Consultation on database structure

Data preparation for statistical analysis


Online surveys (CATI, CAWI, and others), eCRF

Statistical Analysis:

Graphic presentation of results: figures, boxplots, charts


Structural equation modelling (SEM)

Parametric and nonparametric tests and post-hoc analyses

Preparation of research reports

Presentation of results following the standards of Philadelphia-listed journals



Involvement of BioStat experts with academic doctoral degrees.

Work based on Cloud Computing technology.

Utilization of advanced statistical packages like R and SPSS,
as well as the Python programming language.

Analyses in accordance with the requirements
of ISI Master Journal List.

Capability to conduct analyses and prepare reports in English.

12-month guarantee (e.g. assistance with peer review).
We offer our clients comprehensive statistical services for both commercial and non-commercial clinical trials as well as observational studies conducted on behalf of entities in the pharmaceutical, medical, biotechnological industries, and research institutions.
We execute research projects across various therapeutic areas. In our daily work, we utilize the following programs: the R package, SPSS, the Python programming language, and bioinformatics tools (Blast, Phred/Phrap, Bioconductor).
The Biostat team consists of experts in biostatistics, mathematics, bioinformatics, and data management, whose analyses are published in journals on the Master Journal List.
Oncologist
Biostat's support in preparing statistical reports of research results is immensely valuable. I have used Biostat's assistance several times to analyse data. The employees of Biostat distinguish themselves with a high level of knowledge and competence, showing openness and readiness to solve problems and doubts.
Motor Preparation Trainer, Representative of Poland in Rugby 7.
I was greatly impressed by the help, the opportunity for phone consultations, and the clear explanation of the tools used for my doctoral work. It is a pleasure to collaborate with people who are 200% committed and passionate about their work.

Verification of Statistical Hypotheses

Development of the Statistical Section of the Study Protocol

Sample size calculation, power simulations, statistical justifications

Selection of variables and measurement scales

Choice of statistical methods and tests

Statistical modeling

Development of a Randomization List, Electronic Randomization System (Interactive Web Response System, IWRS)

Preparation of a Statistical Analysis Plan, development of table, listing, and graph shells
Statistical Analyses:

Comparison of efficacy for dichotomous and continuous endpoints

Comparison of efficacy for dichotomous and continuous endpoints in the presence of confounding factors (ANCOVA, logistic regression models, Poisson regression models)

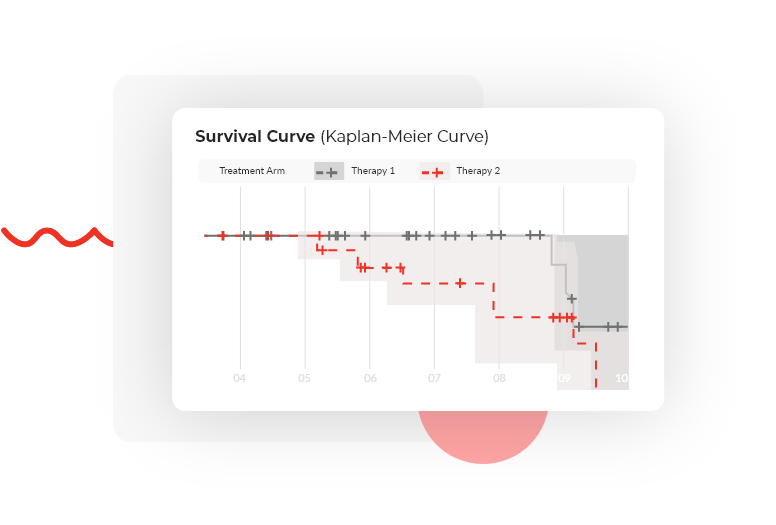
Survival analyses

Longitudinal study analyses, mixed models, hierarchical analyses

Safety analyses and adverse event coding using the MedDRA Dictionary (Medical Dictionary for Regulatory Activities)

Big data analyses

Preparation of a Statistical Report, assistance in the preparation of a Clinical Study Report (CSR)

Support in the preparation of publications
We present each statistical analysis in the form of a report that addresses the research questions provided by the client. The report developed by us always includes:

Methodology section

Tables with numerical results

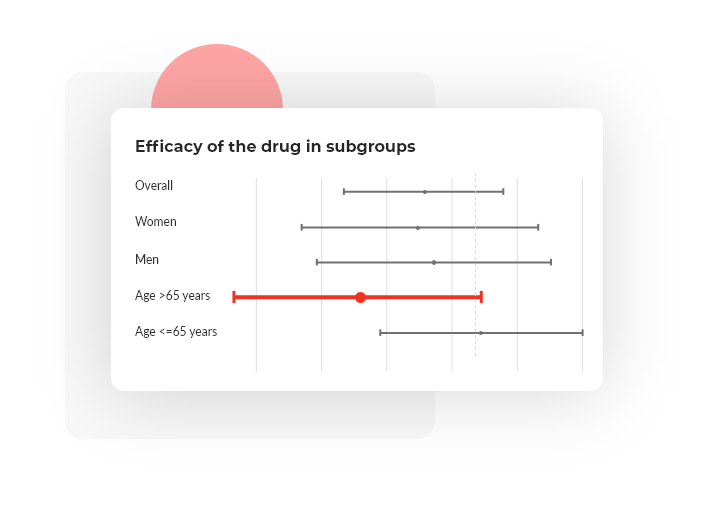
Visualization of results in the form of high-quality graphs
We carry out both simple analyses and more complex research projects. Reports are tailored to the client's needs and the statistical analysis plans created in cooperation with the client. For statistical tests, we always report easy-to-interpret p-values.
Statistical Reports:

Reports with detailed statistical interpretation

Reports in English

Bilingual reports
Don't buy a pig in a poke! Sample reports:


Message
Send us an email describing your research problem. It's best if it includes attachments such as a database, a set of hypotheses, or an inspirational article.

Specification of requirements
If we find that your initial message doesn't provide sufficient information for us to prepare a pricing, we will inquire for more details.


Analysis plan and cost estimation
Remember that this is our proposal, and you are not obliged to accept it. If the cost exceeds your financial capabilities, we can always limit the scope of the analysis – just talk to us.

There were 3.6% reoperations for bleeding: aortotomy place (n = 1), epicardial pacing wire placement (n = 3), right lung tear (n = 2), and intercostal vessels (n = 1). The intensive...
MoreViral infections, including cytomegalovirus (CMV) and Epstein-Barr virus (EBV), play an important role in carcinogenesis and can influence patients’ prognosis and condition during...
MoreAbstract thinking belongs to intellectual abilities of the highest level of the evolutionary development, thanks to which operations such a classification, systematisation and comparison...
MoreSurgical site infections (SSI) occur in 1.8%–9.2% of women undergoing cesarean section (CS) and lead to greater morbidity rates and increased treatment costs. The aim of the study...
MoreOcena skuteczności i bezpieczeństwa oraz stopnia przestrzegania przez pacjentów dyscypliny leczenia połączenia oksykodon/nalokson (Targin) po rotacji z wcześniej stosowanego...
MoreThe most frequent physical features associated with Turner syndrome is short stature. The main goal of the research was to estimate the height of women with Turner syndrome and to analyze...
MoreIn this paper we consider some sign–changing Lyapunov function in research on regularity and sharply-week regularity of sets of linear extensions of dynamical systems. By regularity...
MoreIn this paper we consider some sign-changing Lyapunov function in research on regularity of sets of linear extensions of dynamical systems on a torus. ...
More
